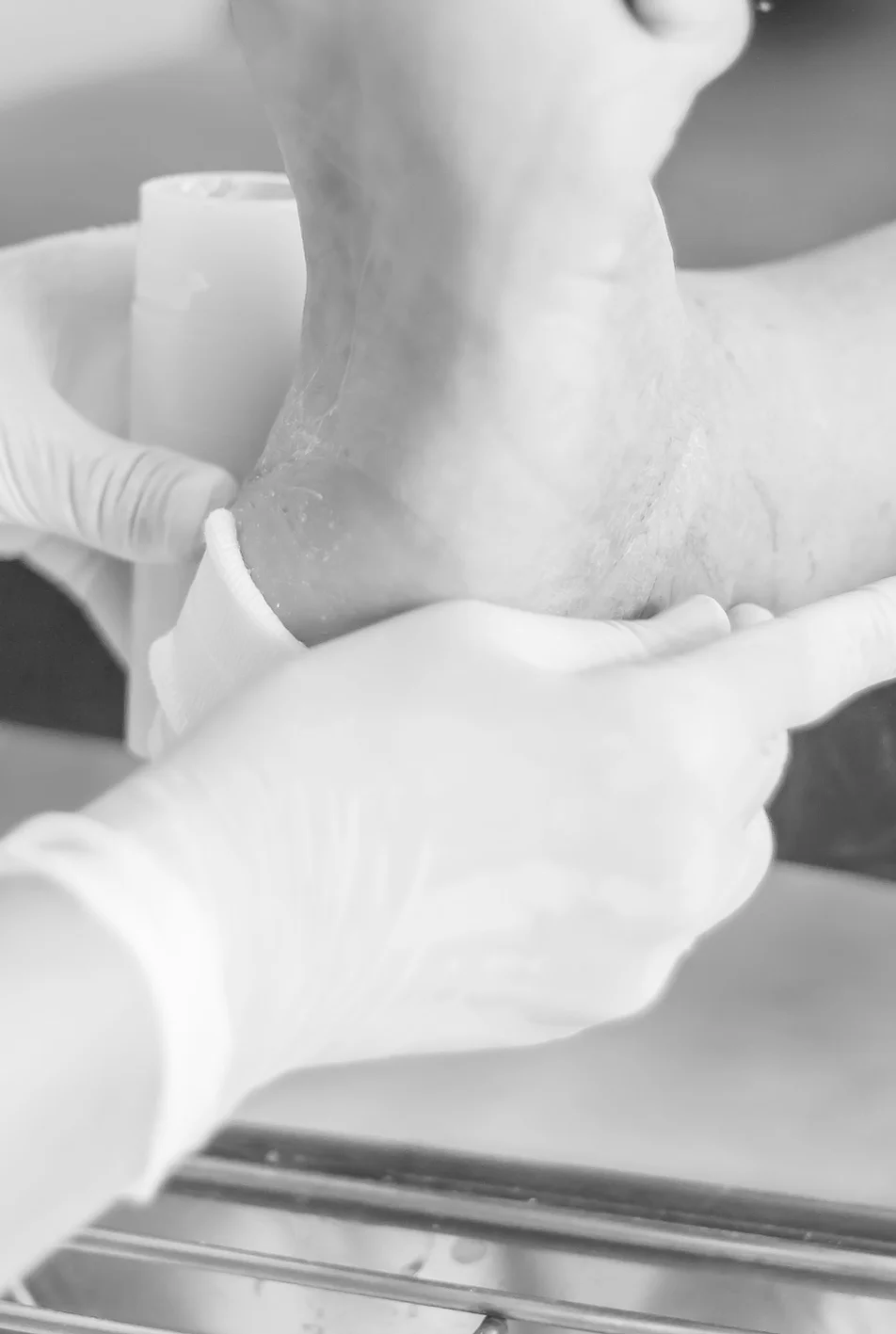
The membrane wrap is a unique DUAL- LAYERED dehydrated human amnion membrane (dHAM) allograft composed primarily of a connective tissue matrix. It is minimally manipulated, preserving the properties that it exhibits in its natural state¹,².
[1] Curr Eye Res. 2000 Mar;20(3):173-7
[2] Placenta 103 (2021) 104–119
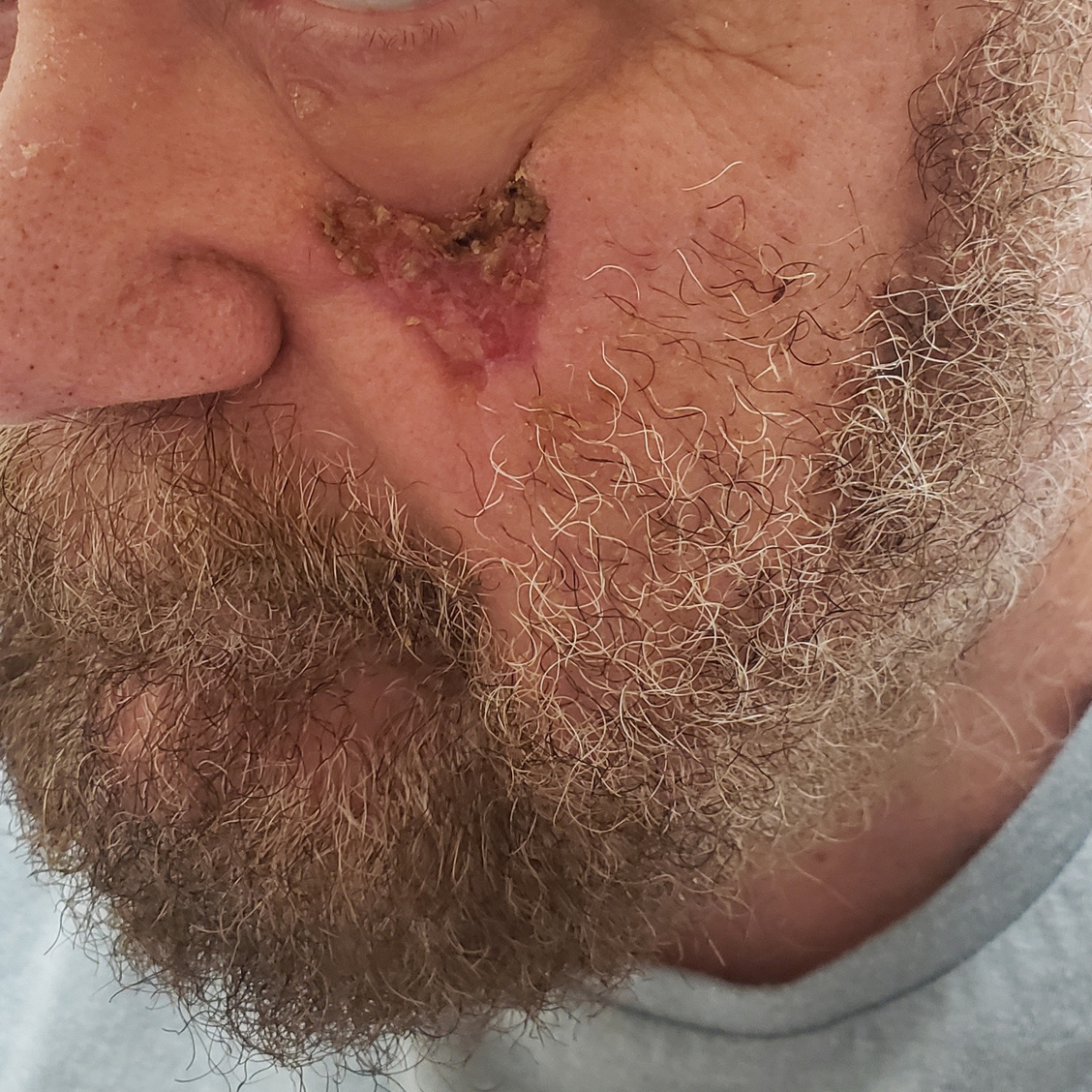
Dressings are easy to apply and remove and are suitable for a wide variety of wounds:
| Amniotic Dual Layer Membrane Wrap Size (cm) | Wound Coverage |
|---|---|
| 1x1 | 1 cm2 |
| 2X2 | 4 cm2 |
| 2X3 | 6 cm2 |
| 4×4 | 16 cm2 |
| 4×6 | 24 cm2 |
| 4×8 | 32 cm2 |
| 6×8 | 48 cm2 |
| 8×8 | 64 cm2 |
| 10x10 | 100 cm2 |
| 20x24 | 480 cm2 |
World’s first wound dressing products that are impregnated with copper oxide microparticles.
Leveraging copper’s potent antimicrobial properties, copper-based wound dressings provide a unique proposition to address acute, critical and chronic wounds. The copper-based wound dressings follow the successful commercialization of copper compounds impregnation into various fabrics and polymers after 15 years of research and protected by multiple patents worldwide.
Dressings are single-use wound dressing with an internal absorbent layer and one or two external non-woven, non-adherent layers. All layers are impregnated with copper oxide particles. The dressings are non-irritant, non-sensitizing, and are available in 7 configurations, with or without an adhesive contour.
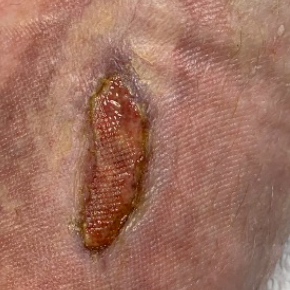
Dressings are easy to apply and remove and are suitable for a wide variety of wounds:
| Two Layers | |
|---|---|
| Antimicrobial Dressing with Copper Oxide | |
| Centimeters | Inches |
| 5 x 6 cm | 2” x 2.4” |
| 10 x 12 cm | 4” x 4.5” |
| 10 x 20 cm | 4” x 8” |
| 20 x 20 cm | 8” x 8” |
| Two Layers with Adhesive | |
|---|---|
| Antimicrobial Dressing with Copper Oxide and an Adhesive Contour | |
| Centimeters | Inches |
| 10 x 10 cm (Internal 5 x 5) | 4” x 4” (Internal 2 x 2.2) |
| 10 x 25 cm (Internal 5 x 20) | 4” x 10” (Internal 2 x 7.8) |
| Three Layers | |
|---|---|
| Antimicrobial Dressing with Copper Oxide – appropriate for tunnels and deep wounds | |
| Centimeters | Inches |
| 10 x 12 cm | 4” x 4.5” |
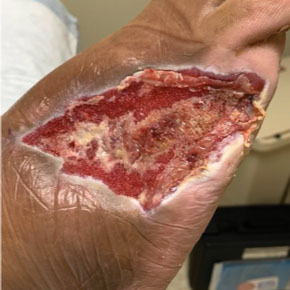
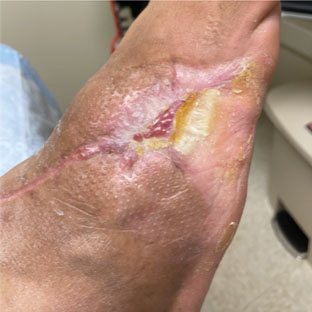
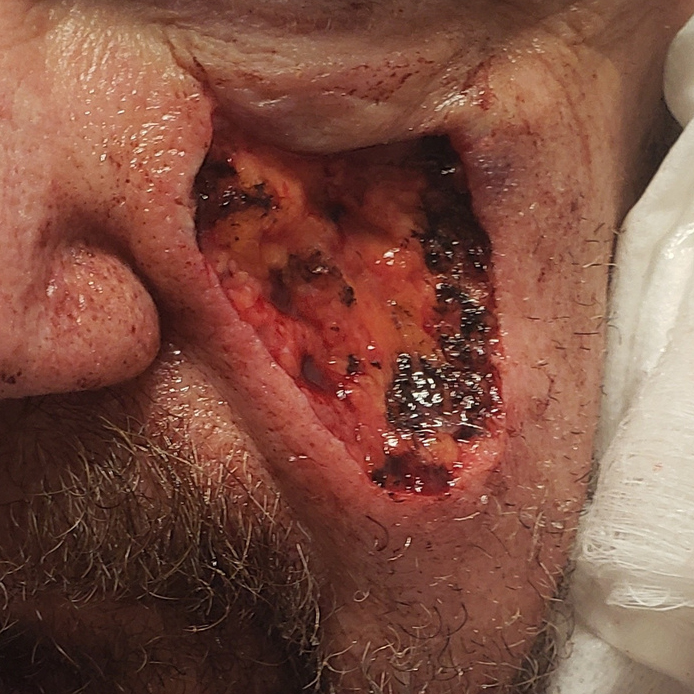
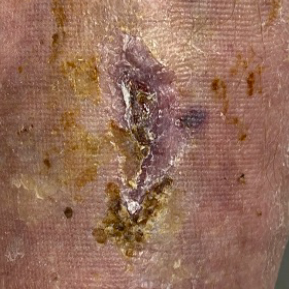
Dressings are easy to apply and remove and are suitable for a wide variety of wounds:
Terms and conditions / Privacy Policy
© Copyright by CSQ Bio 2024